In the ever-expanding market of health supplements, consumers and industry professionals alike face the challenge of verifying product legitimacy. One critical tool in this verification process is the ability to look up health food approval numbers, which serve as official government endorsements of a product's safety and claimed benefits. The pathways to access this information, however, vary significantly across regions and regulatory bodies, creating a complex landscape that requires careful navigation.
Understanding the importance of health food approval numbers begins with recognizing their role in consumer protection. These unique identifiers, often printed on product packaging, represent a supplement's compliance with stringent testing and documentation requirements. Regulatory agencies assign these codes only after thorough evaluation of scientific evidence supporting ingredient safety and functional claims. In markets like China, the "Blue Hat" certification system provides a clear example of how such numbering helps distinguish approved products from unregulated alternatives.
The process of searching for approval numbers typically starts with identifying the relevant regulatory authority for a given product's target market. In the United States, the Food and Drug Administration maintains databases for dietary supplements, though the system operates differently than the pre-market approval processes found in many Asian countries. European Union nations follow yet another framework, with the European Food Safety Authority playing a central role in health claims assessment.
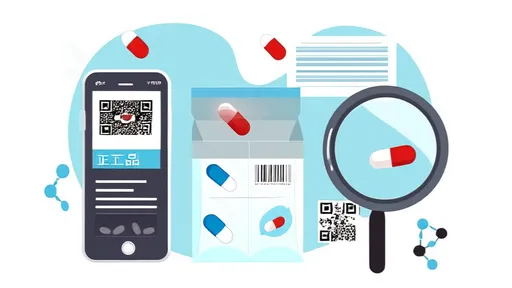
Digital verification platforms have revolutionized how consumers and businesses authenticate health supplements. Many regulatory bodies now offer online databases where users can input approval numbers to access detailed product information. These portals often reveal manufacturing dates, approved health claims, and even past compliance issues. Some Asian markets have taken this further by developing mobile applications that allow consumers to scan QR codes on packaging for instant verification.
Challenges persist in cross-border verification, as approval numbers from one jurisdiction typically hold no weight in another. This becomes particularly problematic for imported health products or online purchases from international sellers. Industry experts recommend checking not just the approval number's validity but also whether the product matches exactly what was registered - including formulation, dosage, and manufacturer information.
The consequences of overlooking proper verification can be severe. Beyond the obvious health risks of counterfeit supplements, businesses face legal repercussions for distributing unapproved products. Recent crackdowns in multiple countries have shown regulators becoming increasingly sophisticated at identifying falsified approval numbers and taking enforcement action against violators.
Emerging technologies like blockchain are beginning to enter this space, with pilot programs testing decentralized verification systems that could make approval number lookup more transparent and tamper-proof. While still in early stages, these innovations promise to address current gaps in the system, particularly for global supply chains where products change hands multiple times before reaching consumers.
For consumers overwhelmed by the technical aspects of approval number verification, several independent organizations have emerged as trusted resources. These third-party verifiers maintain their own databases and often provide simplified explanations of regulatory status. However, experts caution that even these services should be vetted, as some may have conflicts of interest or incomplete information.
The future of health food approval systems appears headed toward greater international cooperation, with discussions underway about potential harmonization of standards. Such developments could eventually simplify the lookup process while maintaining rigorous safety protocols. Until then, understanding the current patchwork of national systems remains essential for anyone involved in the health supplement industry.
Practical tips for effective approval number verification include checking for consistency across all product documentation, being wary of numbers that follow unusual formats, and consulting multiple sources when possible. Regulatory agencies frequently update their databases, so information that was accurate months ago might no longer reflect current status. Setting up alerts for changes to a product's approval status can help both consumers and businesses stay informed.
As the global demand for health supplements continues to grow, so does the importance of robust verification systems. The humble approval number, often overlooked by casual consumers, represents a critical line of defense against an increasingly sophisticated array of counterfeit and substandard products. Mastering the various lookup methods available today is not just good practice - for many in the industry, it's becoming an essential business skill.

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025

By /Jul 28, 2025
By /Jul 28, 2025

By /Jul 28, 2025